Detoxification is often treated as an event, something you do for a few days with supplements, fasting, or cleanses. But true detox is not an event. It is a continuous cellular process. Every moment, cells generate waste: damaged proteins, spent enzymes, oxidized lipids, microbial fragments, and metabolic byproducts. Health depends on the ability to package, move, and remove this material efficiently.
When intracellular clearance pathways are blocked, detox efforts fail, not because the body can’t release toxins, but because the exits are jammed. In these conditions, mobilizing toxins can worsen symptoms as waste circulates without an outlet.
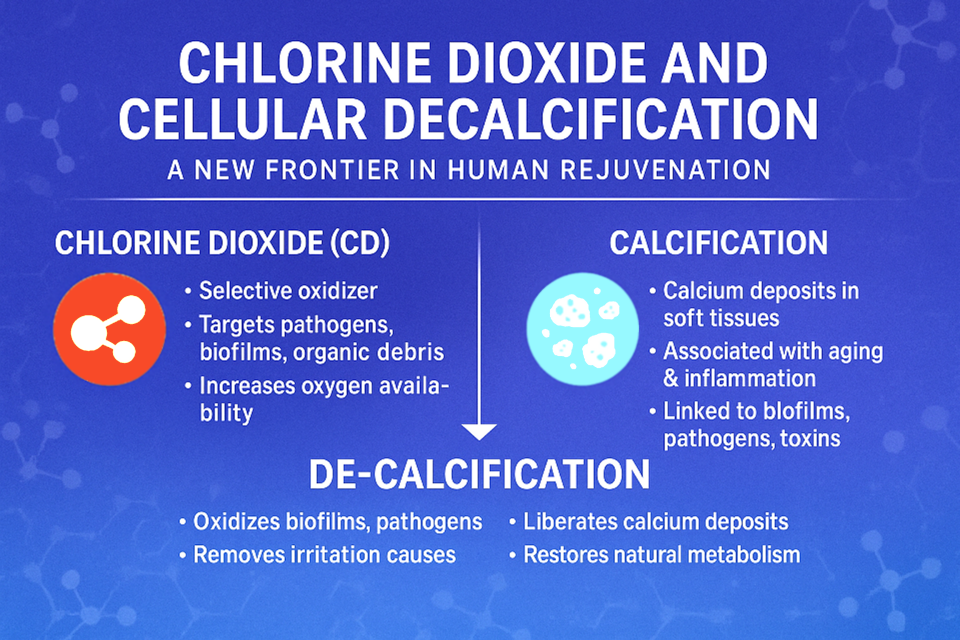
Chlorine dioxide (CD/CDS) is being explored in alternative health research as a supportive upstream intervention, not as a detox agent itself, but as a way to reduce the interference that blocks intracellular waste clearance.
This article explains why detox stalls at the cellular level and how clearing obstructions may restore the body’s natural exit routes.
- What Is Intracellular Waste Clearance?
Intracellular waste clearance is the set of processes cells use to:
-
- identify damaged or spent components
- package waste safely
- move it out of the cell
- deliver it to detox systems (lysosomes, autophagy, lymph, liver, kidneys)
Key clearance mechanisms include:
-
- autophagy (self-cleaning and recycling)
- lysosomal digestion
- proteasomal degradation
- vesicular transport
- membrane export pathways
When these systems function well, cells remain clean, responsive, and efficient. When they stall, waste accumulates and cellular performance declines.
- Why Does Detox Fail When Pathways Are Blocked?
Detox fails not because toxins don’t move, but because they have nowhere to go.
Common blockers include:
-
- Biofilms and Microbial Debris
Biofilms trap waste inside and around cells, clogging exit routes.
-
- Chronic Inflammation
Inflammatory signaling diverts cellular energy away from cleanup.
-
- Oxidative Backlog
Excess waste overwhelms lysosomes and proteasomes.
-
- Iron and Metal Mismanagement
Misplaced metals catalyze oxidative damage that jams clearance systems.
-
- Poor Oxygen Delivery
Autophagy and lysosomal function require stable oxygen availability.
-
- Mitochondrial Inefficiency
Low ATP means cleanup is deprioritized.
-
- Lymphatic Congestion
Even when waste exits the cell, it cannot leave tissues efficiently.
In these conditions, detox protocols mobilize toxins faster than the body can clear them, producing headaches, fatigue, nausea, flares, and “detox reactions.”
- Who Is Most Affected by Blocked Clearance Pathways?
Individuals who experience:
-
- severe reactions to detox or fasting
- worsening symptoms during cleanses
- chronic fatigue after “healing” protocols
- brain fog with detox attempts
- inflammatory flares during supplements
- chemical sensitivity
- poor tolerance to exercise or sauna
- slow recovery after illness
These patterns often indicate clearance congestion, not toxicity alone.
- Where Does Chlorine Dioxide Fit In?
Chlorine dioxide does not act as a chelator, binder, or forced detox agent.
Its proposed role is interference reduction:
-
- Lowering Microbial Load
Fewer microbes = less ongoing intracellular debris.
-
- Weakening Biofilms
Clears physical and biochemical blockages around cells.
-
- Reducing Oxidative Waste
Simplifies the intracellular waste burden.
-
- Supporting Oxygen Balance
Improves energy availability for cleanup processes.
-
- Reducing Inflammatory Noise
Allows cells to reprioritize maintenance and repair.
-
- Supporting Mitochondrial Recovery
Provides ATP needed for autophagy and export.
Chlorine dioxide helps reopen cellular exits by removing what blocks them.
- When Does Intracellular Clearance Restart?
Clearance often resumes:
-
- after chronic infections are reduced
- after biofilm burden declines
- after inflammation subsides
- after mitochondrial efficiency improves
- after lymphatic flow increases
- after iron and oxidative stress normalize
Many people notice delayed but dramatic improvements once pathways reopen, better energy, clearer thinking, reduced sensitivity, and smoother detox responses.
How Chlorine Dioxide May Support Intracellular Waste Clearance
- Reducing Continuous Waste Input
Fewer microbes generate less debris for cells to manage.
- Clearing Biofilm “Exit Jams”
Biofilm reduction improves diffusion and transport.
- Lowering Oxidative Pressure
Cleaner environments prevent clearance system overload.
- Supporting Autophagy Efficiency
Energy and oxygen availability improve recycling.
- Restoring Lysosomal Function
Reduced waste burden allows proper digestion.
- Improving Cellular Export
Cleaner membranes and signaling improve vesicle transport.
- Supporting Downstream Drainage
Lymphatic and liver pathways can keep pace with output.
Detox Is a Flow Problem, Not a Force Problem
True detoxification is about flow, not intensity. When intracellular exits are blocked, forcing detox creates suffering. When exits reopen, detox becomes gentle, continuous, and almost unnoticed. This reframes detox from a battle into a maintenance function, the way a healthy body naturally operates.
Quick How-To Guide
- Remove Interference Before Mobilizing Toxins
Clean first, then detox.
- Support Energy Production
Mitochondrial efficiency precedes clearance.
- Prioritize Hydration and Minerals
Transport requires fluid balance.
- Support Lymphatic Flow
Movement and drainage are essential.
- Introduce Detox Gradually
Let pathways reopen before increasing load.
- Observe Subtle Improvements
Signs of restored clearance include:
-
- fewer detox reactions
- steadier energy
- clearer cognition
- improved tolerance to fasting or supplements
- reduced inflammation
Disclaimer
This article is for informational and research purposes only. It does not diagnose, treat, cure, or prevent disease. Chlorine dioxide is not approved for internal therapeutic use by regulatory agencies. Detoxification is complex; consult qualified professionals before making health-related decisions.